Abstract
Background: Epcoritamab (Epco) is a CD3 bispecific antibody that induces selective, potent T-cell-mediated killing of CD20-positive malignant B-cells. In the phase 1/2 monotherapy study, subcutaneous administered Epco showed a favorable safety profile and promising efficacy, including complete responses in heavily pretreated patients with relapsed / refractory non-Hodgkin's lymphoma (Hutchings et al, Lancet 2021; Thieblemont et al, EHA 2022, LB2364; EPCORE NHL-1). Ongoing clinical studies combining Epco with standard-of-care therapies including combinations with Rituximab show promising preliminary anti-tumor activity in diffuse large B-cell lymphoma and follicular lymphoma (Falchi et al, ASCO 2022 abstracts 7523 and 7524; EPCORE NHL-2 arm 1 and 2; NCT04663347). Here we evaluated the competition for CD20 binding and functional interactions between these agents and modeled its impact on Epco, T-cell, and tumor cell trimer formation that drives Epco clinical activity.
Aims: Investigate the preclinical binding and functional interaction between Epco and Rituximab to establish the potential for the combination of these agents in the treatment of B-cell NHL.
Methods: Competition between Epco and Rituximab for binding to CD20 cells was assessed by Flow Cytometry after incubating the single labeled agent in the presence of increasing concentrations of the competitor and vice-versa (across 0.1 - 300 ug/ml evaluated for each agent), on a range of CD20 expressing lymphoma cell lines. Epco-induced-T cell activation and redirected T cell cytotoxicity was determined by co-culturing T cells with lymphoma cells with varying expression of CD20. Rituximab antibody-dependent cellular cytotoxicity (ADCC) activity was assessed by incubating fresh peripheral blood mononuclear cells with lymphoma cells in the presence of a CD3 control Epco antibody (DuoBody-ctrlxCD20). Data from binding and cytotoxicity was used to quantify the degree of binding curve shifts at different Rituximab:Epco concentrations as well as the impact on predicted trimer formation and cytotoxicity, based on modeling.
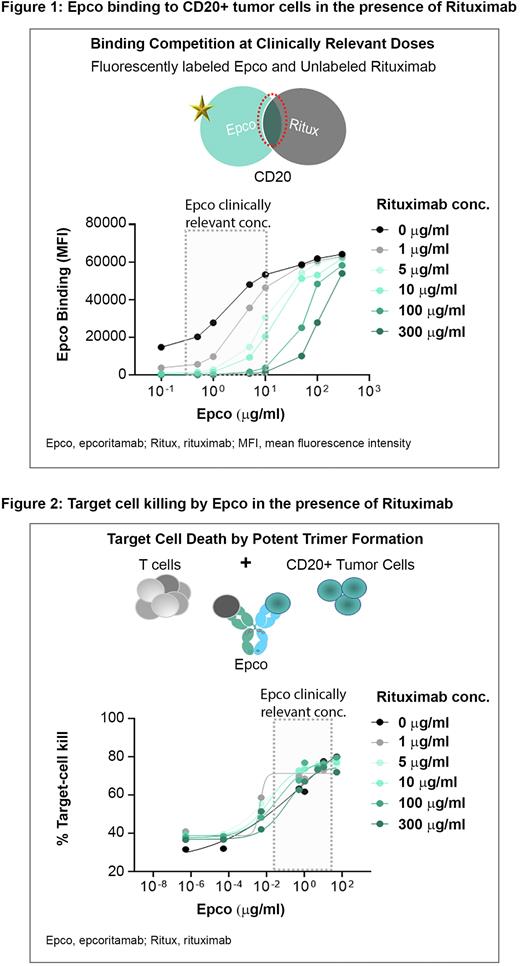
Results: In binding assays, the addition of Rituximab resulted in a dose-dependent reduction of Epco binding to CD20 expressing lymphoma cell lines, with a significant reduction of Epco binding observed at clinically relevant Epco concentrations in cells with high CD20 expression levels (Figure 1). Similar binding interference interactions were observed for binding of Epco to cell lines with low and intermediate CD20 expression levels. Binding of Rituximab to cell lines expressing high CD20 levels was not significantly reduced by clinically relevant concentrations of Epco.
In Epco functional studies, the addition of increasing concentrations of Rituximab did not affect Epco cytotoxic activity at clinically relevant range of Rituximab doses tested (Figure 2). Similarly, no effect of Rituximab on Epco-mediated T cell activation was observed at clinically relevant concentrations of Rituximab and Epco.
ADCC activity at clinically relevant concentrations of Rituximab (10-250 ug/ml) was not impacted by incubation with the CD3 control Epco antibody (DuoBody-ctrlxCD20), even at concentrations exceeding the Epco clinical concentrations.
Modeling and simulation of in vitro data showed that at clinically relevant concentration of Epco, the presence of clinically relevant concentration of Rituximab does not meaningfully impact Epco trimer formation, and the predicted trimer formation remains above EC90 relative to that needed for 50% cell kill.
Conclusions: Our studies showed that while Rituximab exerts significant interference on Epco binding to CD20-expressing cells, tumor-cell cytotoxicity by Epco is minimally affected by clinically relevant concentrations of Rituximab. This conclusion is supported by preclinical modeling and simulations showing that Epco activity driven by trimer formation is not meaningfully impacted by clinical concentrations of Rituximab. Furthermore, we found a limited effect of Epco on Rituximab binding to CD20 and no impact on ADCC activity by Rituximab, thereby supporting potential synergy in the combination between these agents. Results from this study confirm the ability to combine Epco with CD20 antibodies, specifically with Rituximab, and the potential for synergistic activity of this combination.
Disclosures
Epling-Burnette:AbbVie: Current Employment, Current holder of stock options in a privately-held company, Other: Travel, accommodation, expenses; Merck: Current equity holder in private company; TEVA: Current equity holder in private company; AstraZeneca: Current equity holder in private company. Dandamudi:AbbVie: Current Employment, Current equity holder in publicly-traded company. Konieczna:AbbVie: Current Employment, Current equity holder in publicly-traded company. Calabrese:AbbVie: Current Employment, Current equity holder in publicly-traded company. Wielgos-Bonvallet:Genmab: Current Employment, Current equity holder in publicly-traded company. Kweekel:Genmab: Current Employment. Gresnigt - van den Heuvel:Genmab: Current Employment. Parikh:AbbVie: Current Employment, Current equity holder in publicly-traded company. Li:Genmab: Current Employment. Chiu:Genmab: Current Employment. Chervin:AbbVie: Current Employment, Current equity holder in publicly-traded company. Reilly:AbbVie: Current Employment, Current equity holder in publicly-traded company. Szafer-Glusman:AbbVie: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal